141 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
142 | Add to Reading ListSource URL: consumerhealthchoices.orgLanguage: English - Date: 2013-07-06 14:29:13
|
|---|
143![NDA[removed]DITROPAN (oxybutynin chloride) Tablets (Final Draft submitted[removed]) NDA[removed]DITROPAN (oxybutynin chloride) Syrup (Final Draft submitted[removed]DITROPAN® (oxybutynin chloride) Tablets and Syru NDA[removed]DITROPAN (oxybutynin chloride) Tablets (Final Draft submitted[removed]) NDA[removed]DITROPAN (oxybutynin chloride) Syrup (Final Draft submitted[removed]DITROPAN® (oxybutynin chloride) Tablets and Syru](https://www.pdfsearch.io/img/9230378c54f4bf63e414ebf08d7330b3.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English - Date: 2007-04-09 09:20:37
|
|---|
144![NDA[removed]S-013 Page 3 DITROPAN XL (oxybutynin chloride) Extended Release Tablets NDA[removed]S-013 Page 3 DITROPAN XL (oxybutynin chloride) Extended Release Tablets](https://www.pdfsearch.io/img/adac76496d6446a23d25d8a37bbec62f.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English - Date: 2007-04-09 09:20:38
|
|---|
145 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
146![FOOD AND DRUG ADMINISTRATION Center for Drug Evaluation and Research Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee FDA White Oak Campus, Building 31, The Great Room (Rm[removed]White Oak Co FOOD AND DRUG ADMINISTRATION Center for Drug Evaluation and Research Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee FDA White Oak Campus, Building 31, The Great Room (Rm[removed]White Oak Co](https://www.pdfsearch.io/img/33162c4cd432fe08c5ec1972c892cfa7.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
147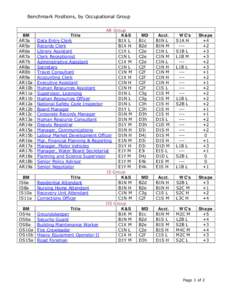 | Add to Reading ListSource URL: www.psc.gov.yk.caLanguage: English - Date: 2014-09-12 18:38:23
|
|---|
148![Microsoft Word[removed]4295b_01_02_Cover Memo_Murphy.doc Microsoft Word[removed]4295b_01_02_Cover Memo_Murphy.doc](https://www.pdfsearch.io/img/4607666cb2a50d7a36f32809abb4418f.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English - Date: 2007-04-09 09:20:36
|
|---|
149 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
150 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|