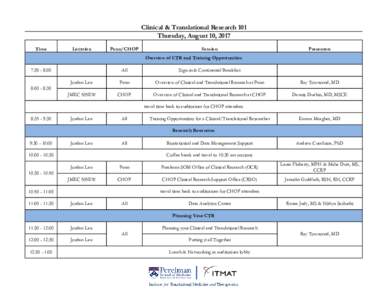
<--- Back to Details
| First Page | Document Content | |
|---|---|---|
 Date: 2014-01-30 11:37:27Clinical research Pharmaceutical industry Food and Drug Administration Design of experiments Institutional review board Clinical trial Public Responsibility in Medicine and Research Investigational device exemption Office for Human Research Protections Clinical investigator Human subject research Covered clinical study |
Add to Reading List |
 § CFR Ch. I (4–1–12 Edition) (3) Requesting that the applicant conduct additional independent studies
§ CFR Ch. I (4–1–12 Edition) (3) Requesting that the applicant conduct additional independent studies