<--- Back to Details
| First Page | Document Content | |
|---|---|---|
 Date: 2015-03-19 15:53:21Fixed dose combination Gilead Sciences Organofluorides Non-nucleoside reverse transcriptase inhibitors Bristol-Myers Squibb Reverse-transcriptase inhibitor ATC code J05 Emtricitabine Fixed-dose combination Chemistry Organic chemistry Pharmacology |
Add to Reading List |
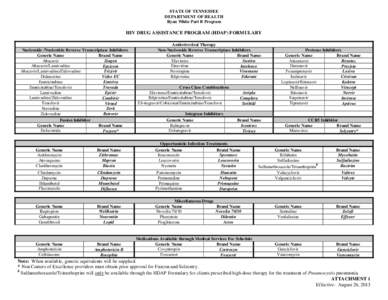
 STATE OF TENNESSEE DEPARTMENT OF HEALTH Ryan White Part B Program HIV DRUG ASSISTANCE PROGRAM (HDAP) FORMULARY
STATE OF TENNESSEE DEPARTMENT OF HEALTH Ryan White Part B Program HIV DRUG ASSISTANCE PROGRAM (HDAP) FORMULARY



