<--- Back to Details
| First Page | Document Content | |
|---|---|---|
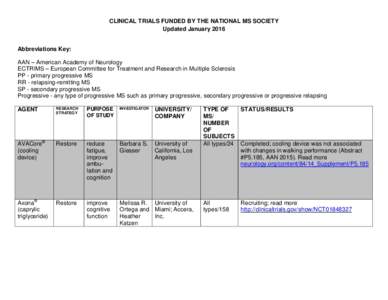 Date: 2016-01-14 16:22:56Clinical trials ClinicalTrials.gov Multiple sclerosis Tecemotide Pseudobulbar affect |
Add to Reading List |
| First Page | Document Content | |
|---|---|---|
 Date: 2016-01-14 16:22:56Clinical trials ClinicalTrials.gov Multiple sclerosis Tecemotide Pseudobulbar affect |
Add to Reading List |