 Date: 2016-02-29 16:05:30Medicine Clinical medicine Anticonvulsants Health GABAA receptor positive allosteric modulators Sodium channel blockers Epilepsy RTT Epileptic seizure Lorazepam Phenytoin Brivaracetam | |  U.S. FDA approves UCB’s new epilepsy treatment, BRIVIACT®, for patients with partial-onset seizures • BRIVIACT® (brivaracetam), a new molecular entity, is indicated as adjunctive therapy in the treatment of partial U.S. FDA approves UCB’s new epilepsy treatment, BRIVIACT®, for patients with partial-onset seizures • BRIVIACT® (brivaracetam), a new molecular entity, is indicated as adjunctive therapy in the treatment of partial
Add to Reading ListSource URL: www.ucb-usa.comDownload Document from Source Website File Size: 99,93 KBShare Document on Facebook
|

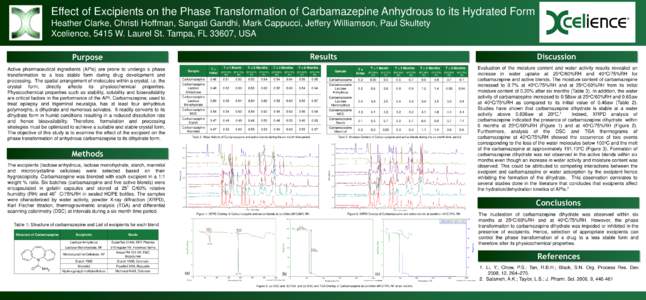



 U.S. FDA approves UCB’s new epilepsy treatment, BRIVIACT®, for patients with partial-onset seizures • BRIVIACT® (brivaracetam), a new molecular entity, is indicated as adjunctive therapy in the treatment of partial
U.S. FDA approves UCB’s new epilepsy treatment, BRIVIACT®, for patients with partial-onset seizures • BRIVIACT® (brivaracetam), a new molecular entity, is indicated as adjunctive therapy in the treatment of partial