<--- Back to Details
| First Page | Document Content | |
|---|---|---|
 Universal identifiers Pharmaceutical industry Drug safety Pharmaceutical sciences Object identifier Medicine International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Electronic Common Technical Document IFPMA Identifiers Clinical research Research |
Add to Reading List |
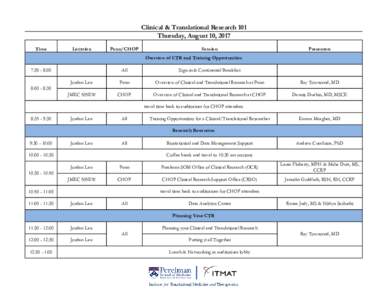
 E2B(R3) OID Information Paper
E2B(R3) OID Information Paper