<--- Back to Details
| First Page | Document Content | |
|---|---|---|
 Date: 2016-08-19 03:14:51Clinical research Clinical data management Electronic common technical document Pharmaceutical industry Pharmaceuticals policy Common Technical Document Marketing authorization Electronic submission Drug Master File |
Add to Reading List |
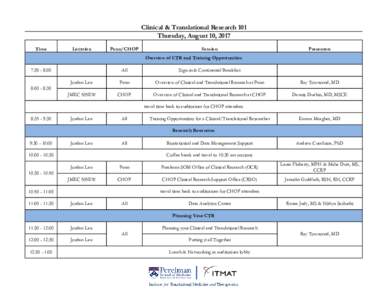
 Practical Guidance For the Paper Submission of Regulatory Information in Support of a Marketing Authorisation Application When Using an eCTD or a NeeS as the Source Submission. v2.0
Practical Guidance For the Paper Submission of Regulatory Information in Support of a Marketing Authorisation Application When Using an eCTD or a NeeS as the Source Submission. v2.0