<--- Back to Details
| First Page | Document Content | |
|---|---|---|
 Substantial equivalence Food and Drug Administration Tobacco industry Regulation of tobacco by the U.S. Food and Drug Administration Tobacco Health policy Pharmacology |
Add to Reading List |
 | September 12, 2018 Japan Tobacco International, USA, Inc. Glenpointe Centre West 500 Frank W. Burr Blvd. #24 Teaneck, NJDear Mr. Jerry Loftin:DocID: 1xVgy - View Document |
 | Notice to Industry: Additional Tobacco Products Now Regulated by the Food and Drug AdministrationDocID: 1xVey - View Document |
 | Tobacco Region Revitalization Commission for the year ended June 30, 2017DocID: 1xUYe - View Document |
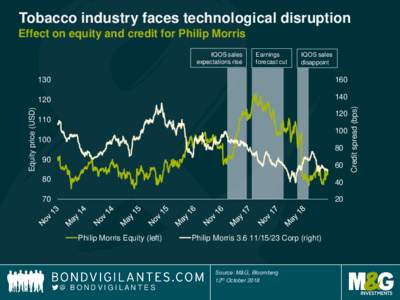 | Tobacco industry faces technological disruption Effect on equity and credit for Philip Morris Earnings forecast cut IQOS salesDocID: 1xUsE - View Document |
 | Illicit Trade in Tobacco Products after Implementation of an FDA Product StandardDocID: 1xTPY - View Document |
 FDAAA Section 913 Supply Chain Security
FDAAA Section 913 Supply Chain Security