1 | Add to Reading ListSource URL: www.2ndchance.infoLanguage: English - Date: 2014-02-04 18:59:14
|
|---|
2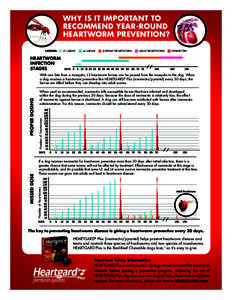 | Add to Reading ListSource URL: joinpowerof12.comLanguage: English - Date: 2014-04-28 09:52:20
|
|---|
3 | Add to Reading ListSource URL: joinpowerof12.comLanguage: English - Date: 2014-02-20 12:25:30
|
|---|
4 | Add to Reading ListSource URL: joinpowerof12.comLanguage: English - Date: 2014-05-07 12:15:00
|
|---|
5 | Add to Reading ListSource URL: www.heartgard.comLanguage: English - Date: 2015-02-13 15:06:22
|
|---|
6![Date of Approval: May 23, 2008 FREEDOM OF INFORMATION SUMMARY SUPPLEMENTAL NEW ANIMAL DRUG APPLICATION NADA[removed] Date of Approval: May 23, 2008 FREEDOM OF INFORMATION SUMMARY SUPPLEMENTAL NEW ANIMAL DRUG APPLICATION NADA[removed]](https://www.pdfsearch.io/img/fcaf8aa8c670126b5974e1dbb8003a79.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
7![Approval Date: February 10, 2003 FREEDOM OF INFORMATION SUMMARY NADA[removed] Approval Date: February 10, 2003 FREEDOM OF INFORMATION SUMMARY NADA[removed]](https://www.pdfsearch.io/img/b475cc456f1de9a930ad52f70390e015.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
8![Approval Date: June 13, 2002 FREEDOM OF INFORMATION SUMMARY Supplemental NADA[removed] Approval Date: June 13, 2002 FREEDOM OF INFORMATION SUMMARY Supplemental NADA[removed]](https://www.pdfsearch.io/img/37d32a3a758ccde380760cedbb690470.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
9![Microsoft Word[removed]007foi.doc Microsoft Word[removed]007foi.doc](https://www.pdfsearch.io/img/66dcc530125743c82f030ae200c6ad92.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
10![FREEDOM OF INFORMATION SUMMARY NEW ANIMAL DRUG APPLICATION NADA[removed]ProHeart 6 (moxidectin) Sustained Release FREEDOM OF INFORMATION SUMMARY NEW ANIMAL DRUG APPLICATION NADA[removed]ProHeart 6 (moxidectin) Sustained Release](https://www.pdfsearch.io/img/ea35c78211db8eeb2d44d651867b5488.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|